CE Certification Bone Fracture Surgery Company – Femur Intramedullary Nail System – AND
CE Certification Bone Fracture Surgery Company – Femur Intramedullary Nail System – AND Detail:
| Diameter(mm) | Length(mm) | ||
| 9 | 170 | 200 | 240(L&R) |
| 10 | |||
| 11 | |||
| 12 | |||
| Diameter(mm) | Length(mm)(L&R) | |||||
| 9 | 320 | 340 | 360 | 380 | 400 | 420 |
| 10 | ||||||
| 11 | ||||||
| 12 | ||||||
γ-II Interlocking Intramedullary Nail
The medical-lateral angle of 5° allows the insertion at tip of the greater trochanter (the proximal diameter is 16.0mm).
Lateral flattening design eases the insertion and reduces the stress ti the lateral cortical bone.
130 collodiaphysial angle(CCD).
The elastic groove tip design.
Ease the insertion.
Reduce the stress concentration.
Distal curved design prevents the nail tip against to the cortical bone.
Reduce the insertion resistance and pain incidence rate.
Lag screw
The lag screw insertion can provide excellent compaction to cancellous bone.
The tip wide surface provides better anchoring.
Especially for osteoporotic patients.
Anti-rotational and stability achieved by one single element.

Locking bolt
Double thread design to reduce the operation time

End cap
0/5/10/15mm length With the suitable length to take it out

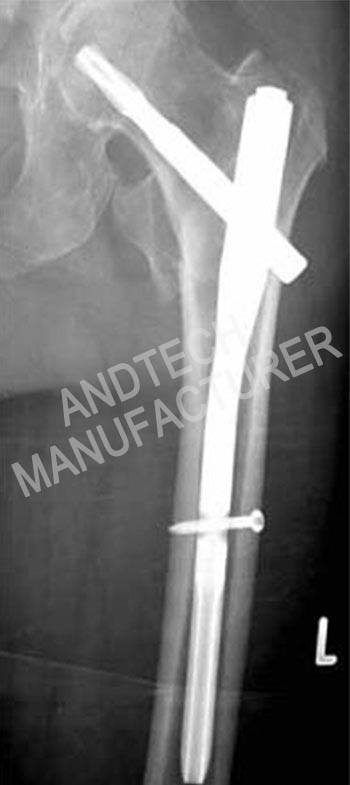
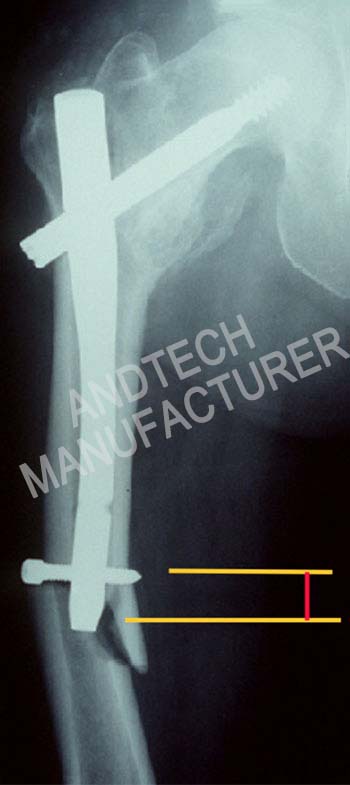
Case

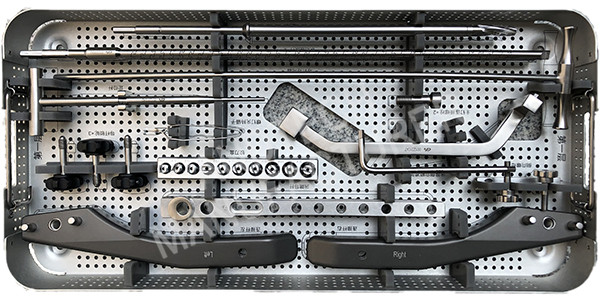
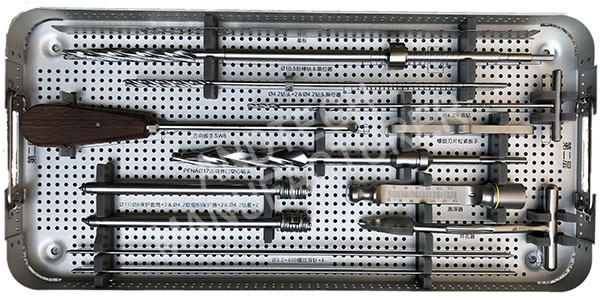
Instruments


Medical Tips
Intertrochanteric fracture
Intertrochanteric fracture (INTERTROCHANTERIC FRACTURE), also known as intertrochanteric fracture.
It is characterized by frequently-occurring elderly people, mostly low-energy injuries caused by osteoporosis in the elderly, and with the trend of aging population, the incidence rate is increasing year by year.
For intertrochanteric fractures, the most commonly used internal fixation materials include intramedullary and extramedullary fixation. Among them, intramedullary fixation is due to.
The surgical incision is small, the bleeding is small, and the operation time is short. It conforms to the principle of minimally invasive. At the same time, intramedullary fixation is more conducive to the conduction of load and avoids extramedullary
The potential fracture risk of uneven screw stress load caused by eccentric fixation is more widely used in clinical practice. More often in intramedullary fixation.
Intertan, Gamma nail, PFN, PFNA (proximal femoral nail antirotation) are used.
In the fixed structure, the main nail of the medullary nail is too thin or the holding force of the head and neck nail is insufficient, the surgical technique apex distance (TAD) does not meet the requirements, the fracture type is extremely unstable, especially the proximal femur lacks effective support, and the rehabilitation is prematurely loaded. When one of the above conditions exists, there is micro-movement between the internal plant and the bone before the fracture is healed. The combined structure of the femoral reconstruction intramedullary nail is similar to the “mechanical connecting rod”, and the connecting rod has a tendency to “rotate” due to eccentric force. The “rotation” trend will cause the two head and neck studs to move in the opposite direction, and the result is the “Z effect” or “reverse Z effect.
Product detail pictures:



Related Product Guide:
Our corporation insists all along the quality policy of "product top quality is base of organization survival; purchaser pleasure will be the staring point and ending of an company; persistent improvement is eternal pursuit of staff" plus the consistent purpose of "reputation very first, purchaser first" for CE Certification Bone Fracture Surgery Company – Femur Intramedullary Nail System – AND , The product will supply to all over the world, such as: Hungary, Danish, Korea, The company has numbers of foreign trade platforms, which are Alibaba,Globalsources,Global Market,Made-in-china. "XinGuangYang" HID brand products sell very well in Europe, America, Middle East and other regions more than 30 countries.
The factory workers have a good team spirit, so we received high quality products fast, in addition, the price is also appropriate, this is a very good and reliable Chinese manufacturers.










